Antibiotic resistance is a serious global health concern that threatens the effective treatment of community- and hospital-acquired bacterial infections in patients of all ages. It occurs when bacteria evolve mechanisms to survive exposure to the antibiotics designed to kill them. This can lead to infections that are harder to treat, longer hospital stays, and increased mortality.
The discovery of antibiotics was and is an evolution that saves lives daily, but their abuse leads to severe consequences and even death. Antibiotics are medicines that fight bacterial infections in people. Understanding the impact of antibiotic resistance is crucial, especially for healthcare professionals. This essay will focus on the causes of antibiotic resistance, economic costs, factors contributing to resistance, the effectiveness of measures taken to prevent it, and new strategies for treating resistant infections.
Microorganisms, such as bacteria, are living organisms that adapt over time. Their main objective is to replicate, survive, and spread as rapidly as possible. As a result, microbes adjust to their surroundings and evolve in ways that guarantee their continued existence. If something stops their ability to grow, such as an antibiotic, genetic modifications may arise, making the bacteria immune to the medication and allowing them to survive. It is the natural process of bacteria to develop drug resistance. However, several elements are currently at stake in the multifaceted etiology of antibiotic resistance. This involves antibiotic overuse and abuse, inexact diagnosis and improper antibiotic prescribing, patient sensitivity loss and self-medication, poor healthcare environments, poor personal hygiene, and widespread agricultural use. (1)
The pharmacology behind antibiotics includes destroying the bacterial cell by either preventing cell reproduction or changing a necessary cellular function or process within the cell. Antimicrobial agents are classically grouped into two main categories based on their in vitro effect on bacteria: bactericidal and bacteriostatic. Common teaching often explains that bactericidal antibiotics “kill” bacteria and bacteriostatic antibiotics “prevent the growth” of bacteria. Levels of resistance may vary greatly within related bacterial groups. Susceptibility and resistance are usually measured as a function of minimum inhibitory concentration (MIC), the minimal concentration of drug that will inhibit the growth of the bacteria. Susceptibility is actually a range of the average MICs for any given drug across the same bacterial species. If that average MIC for a species is in the resistant part of the range, the species is considered to have intrinsic resistance to that drug. Bacteria may also acquire resistance genes from other related organisms, and the level of resistance will vary depending on the species and the genes acquired
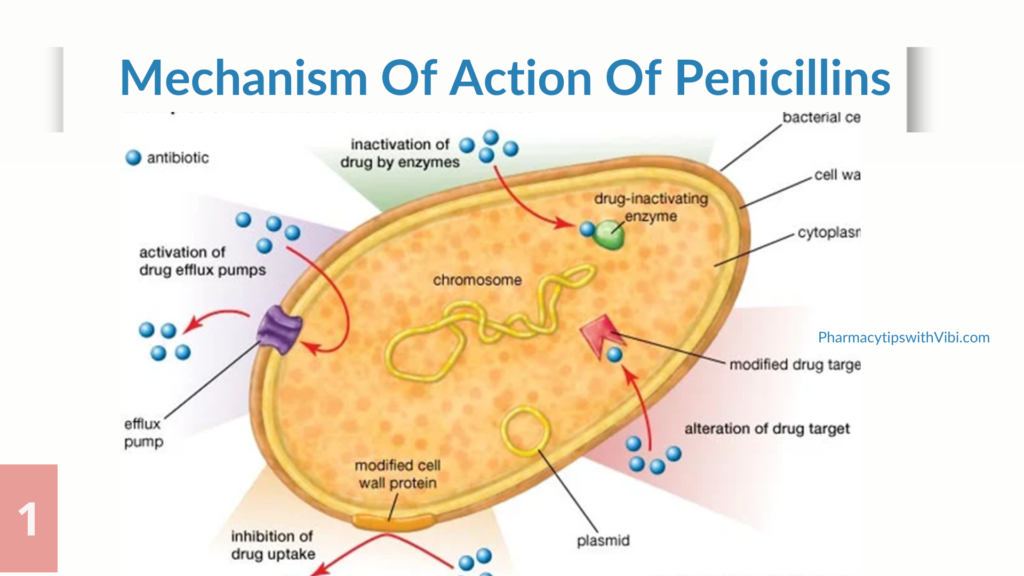
Resistance can be intrinsic: a trait that is shared universally within a bacterial species, is independent of previous antibiotic exposure, and not related to horizontal gene transfer, or resistance can be induced: the genes are naturally occurring in the bacteria, but are only expressed to resistance levels after exposure to an antibiotic. The most common bacterial mechanisms involved in intrinsic resistance are reduced permeability of the outer membrane (most specifically the lipopolysaccharide, in gram-negative bacteria). (2)
Some examples of bacteria with intrinsic antimicrobial resistance are: All gram-positives to aztreonam, Escherichia coli to macrolides, Enterococci to aminoglycosides, cephalosporins, and lincosamides. Resistance could also arise from;
1. Genetic mutation: Changes in a few base pairs during bacterial replication (point mutations) may result in the replacement of one or a few amino acids in a critical target (enzyme, cell wall, or cell structure), as well as control genes or chromosomal structures, resulting in new resistant strains.
2. Selective pressure: Selective pressure may be defined as the environmental conditions that allow the survival and proliferation of organisms with novel mutations or newly developed characteristics. When treated by an antimicrobial, microbes are either destroyed or, if they have resistance genes, they survive. These survivors will multiply, and resistant microbes will rapidly overtake the microbial population as the dominant form.
3. Inaccurate diagnosis: While diagnosing an infection, healthcare professionals sometimes rely on unreliable or inaccurate knowledge, prescribing an antibiotic “just in case” or a broad-spectrum antibiotic when a narrow-spectrum antibiotic might be more appropriate. These circumstances exacerbate selective pressure and hasten antimicrobial resistance.
4. Inappropriate prescription of antibiotics: When doctors are unclear if an infection is exacerbated by bacteria or a virus, they may prescribe antibiotics. Antibiotics, however, do not act against viral infections, and resistance may develop.
5. Self-medication: In Southeast Asian regions, antibiotics are widely used without a physician’s prescription. Self-medication with antibiotics (SMA) is linked to the possibility of improper drug usage, which puts patients at risk for adverse drug reactions, masking signs of underlying diseases, and the development of drug resistance in microbes.
6. Inadequate and overuse of antibiotics: If an individual does not finish a course of antibiotics, some bacteria may thrive and develop resistance to that antibiotic. In 1945, the discoverer of antibiotics, Alexander Fleming, issued a public warning against the overuse of antibiotics, realizing the dangers associated with their inappropriate use. Taking antibiotics too often for the wrong reasons can develop modifications within the bacteria that antibiotics do not work against them.
7. Extensive use in agriculture: In both the industrialized and emerging parts of the world, antibiotics are used as growth supplements and growth promoters for animals. Treatment of livestock with some antibiotics, much like in humans, will result in the appearance of antibiotic-resistant bacteria. The antibiotic-resistant bacteria found in livestock can be pathogenic to humans, readily spread to humans by food chains, and is widely circulated in the ecosystem by animal waste. In humans, this may lead to complex, untreatable, and long-term infections. (3)
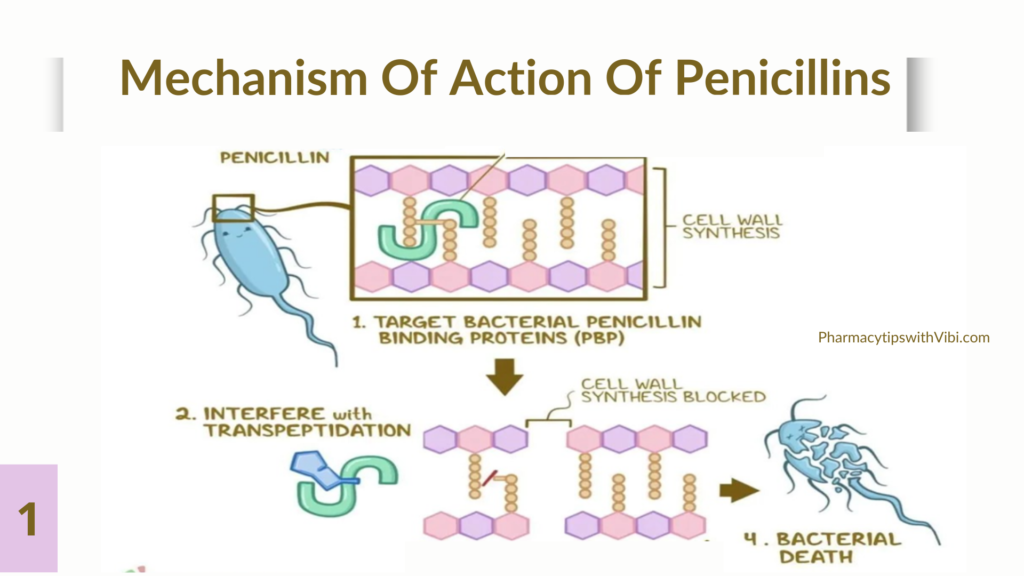
The first antibiotic discovered was a β-lactam, penicillin. The Scottish scientist Alexander Fleming accidentally noticed the production of a substance with antimicrobial properties by the mold Penicillium notatum. Over the last 30 years, many new β-lactam antibiotics have been developed.
By definition, all β-lactam antibiotics have a β-lactam nucleus in their molecular structure. The β-lactam antibiotic family includes penicillins and derivatives, cephalosporins, carbapenems, monobactams, and β-lactam inhibitors. The core compound of penicillin, 6-aminopenicillanic acid (6-APA), is used as the main starting point for the preparation of numerous semi-synthetic derivatives. β-lactam antibiotics work by inhibiting cell wall synthesis by binding to so-called penicillin-binding proteins (PBPs) in bacteria and interfering with the structural cross-linking of peptidoglycans, preventing terminal transpeptidation in the bacterial cell wall. Consequently, it weakens the cell wall of the bacterium and finally results in cytolysis or death due to osmotic pressure. The first bacterial enzyme reported to destroy penicillin was an AmpC β-lactamase of E. coli. Nowadays, bacterial resistance against β-lactam antibiotics is increasing at a significant rate and has become a common problem. There are several mechanisms of antimicrobial resistance to β-lactam antibiotics. The most common and important mechanism through which bacteria become resistant to β-lactams is by expressing β-lactamases. In addition to the production of β-lactamases, resistance can also be due to the possession of altered PBPs. Since β-lactams cannot bind as effectively to these altered PBPs, the antibiotic is less effective at disrupting cell wall synthesis. PBPs are thought to be the ancestors of the naturally occurring chromosomally mediated β-lactamase in many bacterial genera. (1)
The cost of antibiotic resistance can be categorized into three levels: patient, healthcare, and economic:
1. Increased morbidity and mortality: Resistance leads to treatment failures, increasing illness severity and death rates.
2. Extended hospital stays: Ineffective treatments prolong hospitalizations, raising healthcare costs and bed occupancy.
3. Higher healthcare costs: Costs escalate due to longer treatments, additional tests, and the need for newer, more expensive antibiotics.
Antibiotic resistance threatens global health security by compromising our ability to treat infectious diseases effectively. Without effective antibiotics, routine medical procedures such as surgeries, cancer treatments, and childbirth become riskier. (4)
Strategies to combat antibiotic resistance include:
1. Implementing measures to prevent the misuse of current antimicrobials and to stop the spread of infections, as well as eliminating their inappropriate usage: Although the antibiotic itself has a significant impact on antibiotic resistance, how it is utilized also plays a part in its selective role. Synthetic methods like diversity-oriented synthesis, a factor of safety, and common technical documents can be employed to build unheard-of scaffolding that supports the complexity of natural products.
2. Enhancing hygiene and infection control measures in healthcare settings: This includes hand hygiene, sterilization, and isolation protocols to prevent the spread of resistant bacteria (3).
3. Educating healthcare professionals and the public about the dangers of antibiotic resistance: Promoting practices that minimize its spread, such as proper hand washing, vaccination, and stopping auto-medication (especially in the use of antibiotics for treating flu).
4. Encouraging international cooperation: Tackling antibiotic resistance through shared data, resources, and coordinated action plans.
Antibiotic resistance represents a critical threat to public health, requiring urgent attention and concerted action. Understanding the causes and consequences of antibiotic resistance is vital in shaping effective responses to this global challenge. By implementing comprehensive strategies and fostering collaborations, we can mitigate the impact of antibiotic resistance and safeguard the effectiveness of these life-saving medications for future generations.
Bibliographic References
1. Uddin, T.M. Chakraborty, A.J. Zidan,B.R.M. et al. Antibiotic resistance in microbes. History, mechanisms, therapeutic strategies and future prospects. 2021. https://doi.org/10.1016/j.jiph.2021.10.020
2. Reygaert, W. C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiology. 2018. https://doi.org/10.3934/microbiol.2018.3.482
3. Nadgir, Chinmayee A., and Dalia A. Biswas. “Antibiotic Resistance and Its Impact on Disease Management.” Curēus. April 2023. https://doi.org/10.7759/cureus.38251.
Van Hoek, Angela H. A. M., et al. “Acquired Antibiotic Resistance Genes: An Overview.”. Frontiers in Microbiology. Jan. 2011. https://doi.org/10.3389/fmicb.2011.00203.
5. National Library of Medicine. Antibiotics. medlineplus.gov/antibiotics.html.
6. Patel, P., Wermuth, H. R., Calhoun, C., & Hall, G. A. (2023, May 26). Antibiotics. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK535443/
7. Reversing resistance: the next generation antibacterials. Shah NJ. Indian J Pharmacol. 2015;47:248–255.
8. Strategies to combat antimicrobial resistance. Uchil RR, Kohli GS, Katekhaye VM, Swami OC. J Clin Diagn Res. 2014;8:0–4.
9. Pathogen-selective killing by guanylate-binding proteins as a molecular mechanism leading to inflammasome signaling. Feng S, Enosi Tuipulotu D, Pandey A, et al. Nat Commun. 2022;13:4395
