Sodium ion channels play a crucial role in the physiological function of cells, and their deregulation has been implicated in a wide range of diseases and disorders, such as neuropathic, cardiac -arrhythmia, and epilepsy.Voltage-gated sodium channels are transmembrane proteins that open when the membrane potential in their vicinity becomes depolarized, allowing the flow of sodium against its concentration gradient.1
This article will focus on voltage-gated sodium channels since therapy widely uses them. Voltage-gated sodium ion channels are the first channels to open in response to changes in voltage, allowing positively charged sodium ions to accumulate in the interior of the cell. The ability of a cell to depolarize is critical in excitable cells, such as neurons, endocrine cells and muscle cells, where this electrical signal can be used to give rise to an action potential that then transforms into a response like the release of neurotransmitters or contraction, respectively2.
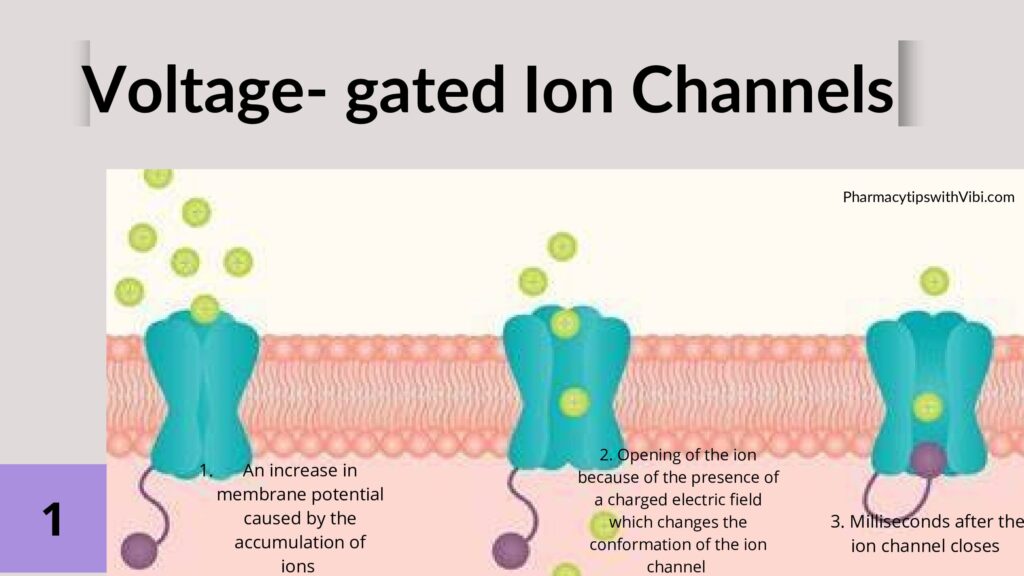
The molecular structure of the voltage-gated sodium channels enables us to understand their functioning and how their channelopathy comes about.
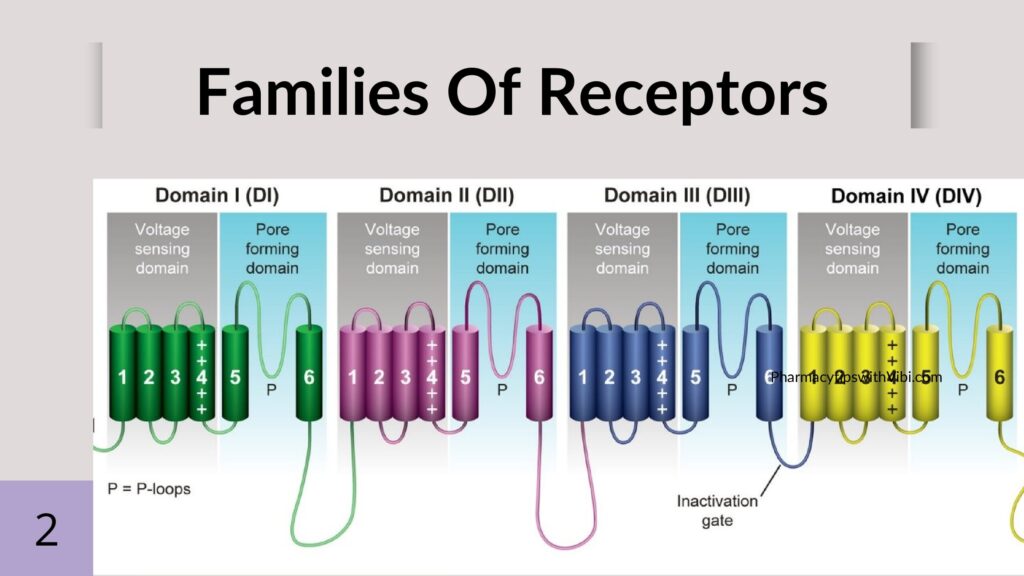
Voltage-gated sodium channels consist of one pore-forming alpha subunit through which the sodium will pass and one to two beta subunits.
Alpha subunits: The nine alpha subunits are made up of four transmembrane domains, with each domain consisting of six segments. The voltage sensory component is on the fourth segment, it contains 5–6 positively charged arginine residues, which are extremely sensitive to changes in the membrane potential of each alpha domain.
Beta subunits: The genes SCN1B–SCN4B encode five identified beta subunits. Interestingly, the beta subunits are actually a part of the Ig superfamily of cell adhesion molecules due to their large, extracellular V-set immunoglobulin (Ig) domain. While the beta subunit does not have a sodium channel pore, it regulates excitability by modulating the localization, gating, and kinetics of the pore on the adjacent alpha subunit.
Voltage-gated sodium channels have two gates: an activating gate that is voltage-dependent and an inactivating gate that is time-dependent. The activating gate allows the influx of sodium and cell depolarization2.
Voltage-gated sodium channels are the target of multiple pharmacological compounds, including anaesthetics, antiepileptics, antiarrhythmics, and in treating some autoimmune diseases.
Systemic administration of the local anaesthetic lidocaine is antinociceptive in both acute and chronic pain states, especially in acute postoperative and chronic neuropathic pain.
These effects cannot be explained by its voltage-gated sodium channel-blocking properties alone, but the responsible mechanisms are still elusive.
Anaesthetics such as lidocaine involve the prevention of nerve conduction and blocking the potentiation of pain signals.
Lidocaine as a local anaesthetic is involves the blocking of voltage-gated sodium channels (VGSCs) leading to a reversible block of action potential propagation. Like with all local anaesthetics, only the un-ionised form of the drug (i.e. the free base form) can permeate the lipophilic cell membranes. Intracellular, the ionised form blocks sodium channels by binding to Segment 6 of Domain 4 of the α-subunit of the ion channel. When the local anaesthetic is bound to the sodium channel, the influx of Na+ is interrupted and action potential generation and propagation are inhibited. Local anaesthetics bind preferably to the open or inactivated state of VGSCs; thus, the onset of local anaesthetic action is faster in rapidly firing neurones3.
Using sodium channels in the treatment of cardiac disorders involves the treatment of arrhythmia using drugs like phenytoin and carbamazepine.
Epilepsy is a neurological condition that causes unprovoked, recurrent seizures. A seizure is a sudden rush of abnormal electrical activity in your brain. Doctors diagnose epilepsy when you have two or more seizures with no other identifiable cause.4
Researchers have identified over 200 missense mutations that affect seven different voltage-gated sodium channels that cause excitability disorders or channelopathies. While these disorders are rare, they show how proteins altered by genetic variability and mutations can profoundly affect the functioning of voltage-gated sodium channels.
One example of such a mutation is the SCN1A gene, which encodes the NAV 1.1 voltage-gated sodium channel alpha subunit. This mutation has been well-studied and has shown to be the etiology for many primary epilepsies, including febrile seizures. Primary epilepsies are epilepsy for which there is no apparent cause, such as cerebral vascular accident, cerebral palsy, or other contributing factors. Since mutated voltage-gated sodium channels are one of the primary causes of epilepsy, it makes sense that many first-line drugs for epilepsy are from the sodium channel blocker class2.
Phenytoin has a highly selective inhibitory effect on the motor area of the cerebral cortex. For its mode of action, phenytoin binds to the inactivated state of the Na+ channel to prolong the neuronal refractory period.
Moreover, it is generally believed that phenytoin exerts its antiepileptic effect by stabilising the function of brain cell membranes and increasing the levels of the inhibitory neurotransmitters serotonin (5-HT) and γ-aminobutyric acid (GABA) in the brain5 .
Using sodium channels in the treatment of cardiac disorders involves in the treatment of arrhythmia using some drugs like disopyramide and flecainide.
A cardiac arrhythmia is simply defined as a variation from the normal heart rate and/or rhythm that is not physiologically justified6.
Dysfunction of NaV1.5 owing to mutations or pathophysiological conditions causes life-threatening cardiac arrhythmias, which are terminated and prevented by treatment with antiarrhythmic drugs.
Although other voltage-gated sodium channels are expressed in the heart, they are unable to compensate for dysfunction of NaV1.5, which highlights the importance of this sodium channel in cardiac physiology and pathophysiology.
There are four classes of antiarrhythmics, based on the Vaughan-Williams (VW) classification system: Class I(being our main focus), sodium channel blockers: These drugs prevent sodium from getting through cell membranes. This can slow electrical impulses in the heart muscle. Examples include disopyramide, flecainide, mexiletine, propafenone and quinidine.
Sodium channels are also used to treat some autoimmune diseases like rheumatoid arthritis. Rheumatoid arthritis occurs when your immune system mistakenly attacks your own body’s tissues.
Unlike the wear-and-tear damage of osteoarthritis, rheumatoid arthritis affects the lining of your joints, causing a painful swelling that can eventually result in bone erosion and joint deformity.
Methotrexate is effective in reducing the signs and symptoms of this disease.
The anti-inflammatory effects of methotrexate in rheumatoid arthritis appear to be related at least in part to the interruption of adenosine and possible effects on other inflammatory and immunoregulatory pathways. The immunosuppressive and toxic effects of methotrexate are due to the inhibition of an enzyme involved in the metabolism of folic acid, dihydrofolate reductase7.
The multifaceted role of voltage-gated sodium ion channels in various diseases, emphasizes their significance as potential therapeutic targets. From neurological and cardiac disorders to autoimmune diseases the modulation of sodium ion channels holds promise for advancing medical treatments. By addressing current challenges and continuing to explore new avenues for intervention, researchers can harness the full potential of sodium ion channels in therapy ultimately improving patient outcomes and quality of life.
Bibliographic References
- “Sodium Channels – Latest Research and News | Nature.” Nature, 21 Mar. 2024, www.nature.com/subjects/sodiumchannels
- Hernandez, C. M., & Richards, J. R. (2023, April 10). Physiology, sodium channels. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK545257/
- Hermanns, H., Hollmann, M. W., Stevens, M. F., Lirk, P., Brandenburger, T., Piegeler, T., & Werdehausen, R. (2019). Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. British Journal of Anaesthesia, 123(3), 335–349. https://doi.org/10.1016/j.bja.2019.06.014
- Pietrangelo, Ann. “What Is Epilepsy?” Healthline, 14 Feb. 2022, www.healthline.com/health/epilepsy.
- Patocka, J., Wu, Q., Nepovimova, E., & Kuca, K. (2020). Phenytoin – An anti-seizure drug: Overview of its chemistry, pharmacology and toxicology. Food and Chemical Toxicology, 142, 111393. https://doi.org/10.1016/j.fct.2020.111393
- Antzelevitch, C., Burashnikov, A. (2013). Mechanisms of Cardiac Arrhythmia. In: Gussak, I., Antzelevitch, C. (eds) Electrical Diseases of the Heart. Springer, London. https://doi.org/10.1007/978-1-4471-4881-4_6
- Johns Hopkins Arthritis Center. “Rheumatoid Arthritis Treatment Options | Johns Hopkins Arthritis Center.” Johns Hopkins Arthritis Center, 14 Apr. 2020, www.hopkinsarthritis.org/arthritis-info/rheumatoid-arthritis/ra-treatment.
- Professional, Cleveland Clinic Medical. “What Are Antiarrhythmics?” Cleveland Clinic, my.clevelandclinic.org/health/drugs/22867-what-are-antiarrhythmics.
- Jiang, D., Shi, H., Tonggu, L., El-Din, T. M. G., Lenaeus, M. J., Zhao, Y., Yoshioka, C., Zheng, N., & Catterall, W. A. (2020). Structure of the cardiac sodium channel. Cell, 180(1), 122-134.e10. https://doi.org/10.1016/j.cell.2019.11.041
- Liu H, Clancy C, Cormier J, and Kass R (2003b). Mutations in cardiac sodium channels: clinical implications. Am J Pharmacogenomics 3, 173–179.
- Sampson KJ, and Kass RK (2011). Antiarrhythmic Drugs. In Goodman & Gilman’s Pharmacological Basis of Therapeutics, pp. 815–848.
- Yu, F. H., & Catterall, W. A. (2003). Overview of the voltage-gated sodium channel family. GenomeBiology.com, 4(3), 207. https://doi.org/10.1186/gb-2003-4-3-207
- Jarecki, B. W., Piekarz, A. D., Jackson, J. O., & Cummins, T. R. (2010). Human voltage-gated sodium channel mutations that cause inherited neuronal and muscle channelopathies increase resurgent sodium currents. ˜the œJournal of Clinical Investigation/˜the œJournal of Clinical Investigation, 120(1), 369–378. https://doi.org/10.1172/jci40801
- Marban, E., Yamagishi, T., & Tomaselli, G. F. (1998). Structure and function of voltage gated sodium channels. Journal of Physiology, 508(3), 647–657. https://doi.org/10.1111/j.1469-7793.1998.647bp.x
