Malaria remains one of the most significant public health challenges worldwide, particularly in tropical and subtropical regions.
The disease poses a considerable health burden, hinders economic development, and exacerbates poverty in affected regions.
Globally, there were an estimated 241 million malaria cases in 2020 in 85 malaria-endemic countries, increasing from 227 million in 2019. Most of this increase came from countries in the WHO Africa Region, which accounted for 95% of the cases.
Malaria deaths increased by 12% globally in 2020 compared with 2019, to an estimated 627,000. (1)
A critical concern in malaria management is the emergence of antimalarial drug resistance, which threatens the efficacy of existing treatments and complicates control strategies.
This essay explores the mechanisms and implications of antimalarial drug resistance, current treatment challenges, and innovative strategies to overcome these obstacles.
Antimalarial drugs
Antimalarial drugs currently used in the clinical treatment of human malaria are categorised based on their structural backbone and apparent action. These classes are:
- Endoperoxides: e.g. artemisinin and its derivatives
- 4-Aminoquinolines : e.g chloroquine
- Aryl-amino alcohols: e.g quinine, mefloquine
- Antifolates: e.g pyrimethamine, proguanil, sulfadoxine
- Naphthoquinones: e.g atovaquone
- 8-aminoquinolines: e.g primaquine, tafenoquine
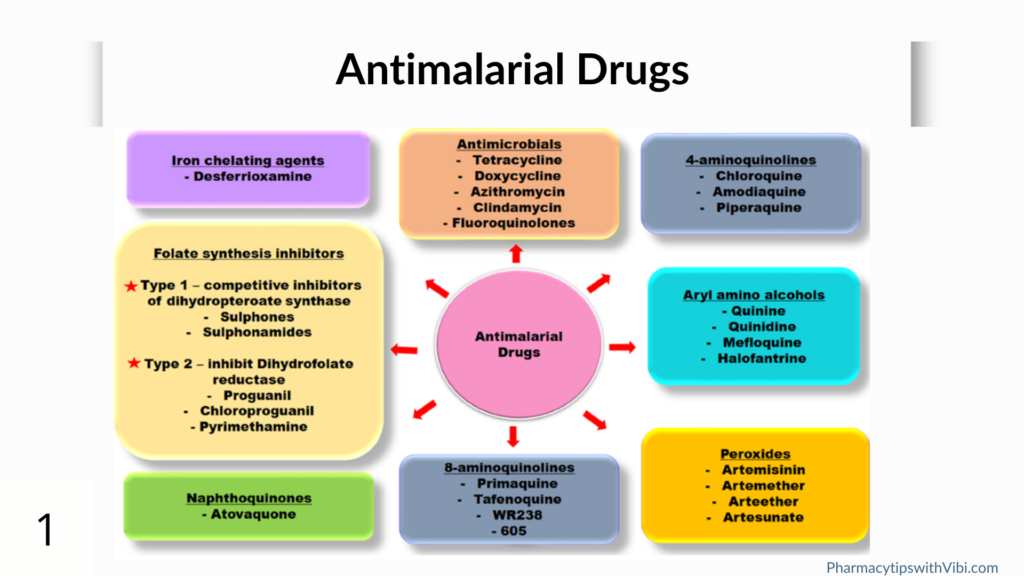
The main targets of inhibition by 4- aminoquinolines, anitifolates and naphthoquinones have been shown to be:
- detoxification of haem released from digested haemoglobin,
- pyrimidine biosynthesis
- mitochondrial cytochrome b involved in oxidoreduction, respectively.
Aryl amino alcohol class inhibitors seem to inhibit the same metabolism as 4-aminoquinolines, whereas endoperoxides, such as artemisinin, act on multiple cellular processes involving reactive oxygen species in Plasmodium cells. (2)
What Is Antimalarial Drug Resistance?
Antimalarial drug resistance is defined as the ability of a parasite strain to survive and/ or multiply despite the administration and absorption of a drug given in doses equal to or higher than those usually recommended, but within tolerance of the subject. (3)
Artemisinin-based combination therapies (ACTs), recommended by WHO for the treatment of uncomplicated malaria is very effective but still partial resistance is observed in the Greater Mekong sub region.
What Is Artemisinin Partial Resistance
Artemisinin partial resistance typically refers to a delay in the clearance of malaria parasites from the bloodstream following treatment with an ACT. As a result, the artemisinin compound is less effective in clearing all parasites within a 3-day period among patients who are infected with artemisinin partial resistant strains of malaria.
Studies have demonstrated that the mechanisms of resistance developed by the parasites against artemisinin compounds affect only one stage of the malaria parasite cycle in humans: the ring stage. It is therefore more appropriate to call the delayed clearance “partial resistance” to highlight this time-limited and cycle-specific feature. It is unknown whether artemisinin partial resistance could further evolve to affect other stages of the parasites, developing into complete resistance. “Full” artemisinin resistance has not been reported.
Currently, even if patients are infected with artemisinin partial resistant parasites, nearly all patients treated with an ACT are fully cured provided that the partner drug is highly efficacious in that geographical area. In the absence of partner drug resistance, artemisinin partial resistance rarely leads to treatment failure. Furthermore, there is no evidence that artemisinin partial resistance alone has resulted in an increase in malaria morbidity and mortality (4).
Causes Of Antimalarial Drug Resistance
Several factors cause antimalarial drug resistance some of which are:
- Spontaneous mutation of parasite: is natural and independent of the drug’s effect.
- Drug accumulation
- Efflux mechanisms (chloroquine, amodiaquine,quinine, halofantrine, mefloquine resistance)
- Decreased affinity of the drug target: which may result frompoint mutations in the respective genes that encode these targets (pyrimethamine, cycloguanil, sulphonamide, atovaquone, artemisinin resistance
- Various species of plasmodium: High rates of transmission and mixed infections can facilitate the spread of resistant genotypes (5).
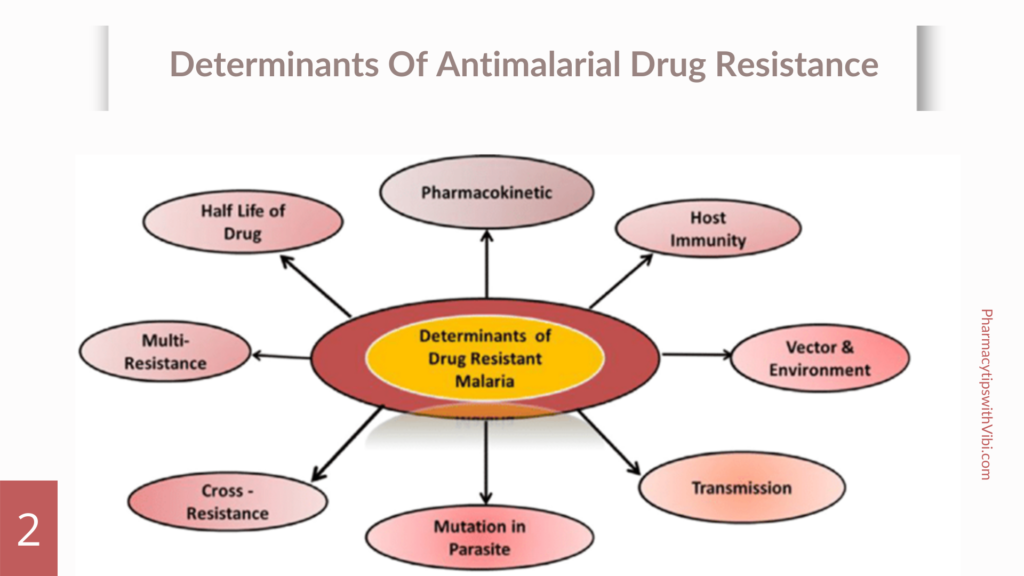
Mechanism Of Antimalarial Drug Resistance
Plasmodium falciparum, the primary parasite causing human malaria, has developed resistance to various antimalarial drugs over time, particularly in endemic regions.
Key mechanisms of resistance include point mutations in the dihydrofolate reductase enzyme, which affect the efficacy of antifolate drugs like pyrimethamine and proguanil.
Additionally, mutations in the pfcrt and pfmdr1 genes enhance the efflux of drugs from the parasite’s digestive vacuoles, contributing to resistance against other antimalarials. Resistance can also arise from gene amplification or regulatory changes that increase the expression of resistant gene products.
Furthermore, Plasmodium species may develop new survival mechanisms, such as acquiring transporters that allow them to utilize host-derived nutrients, potentially leading to resistance against drugs like fosmidomycin. (2)
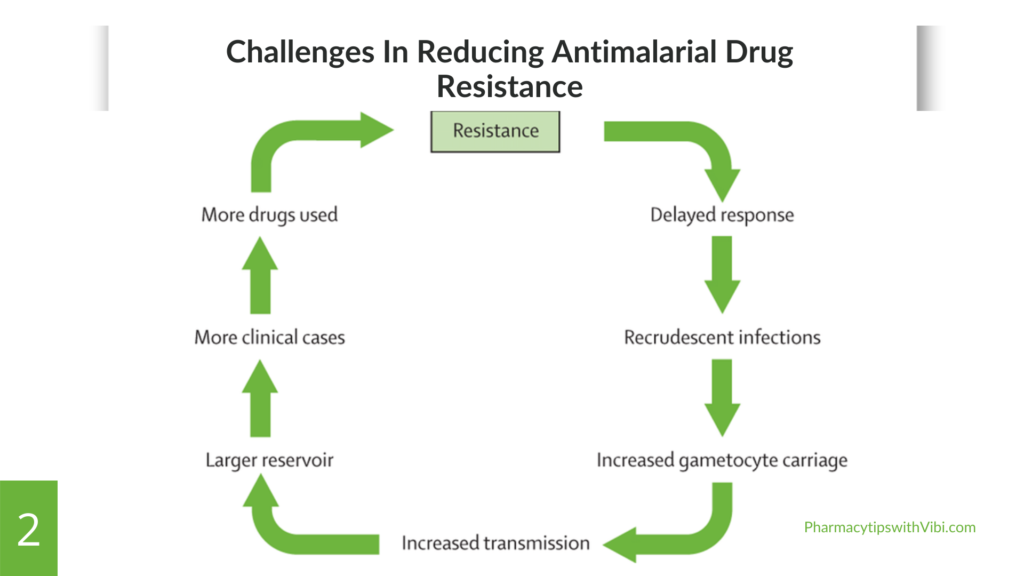
Challenges in Combatting Antimalarial Drug Resistance
The rising challenges to reduce malaria in malaria-endemic areas is as a result of:
- The genetic diversity of parasites allows rapid adaptation of resistance mechanisms. This diversity complicates the identification of effective treatment protocols.
- Reduced effectiveness of existing antimalarial drugs due to resistance results in limited treatment options.
- The inappropriate use of antimalarial drugs or counterfeit drugs and lack of awareness contributes to the development and spread of resistance.
- Resistance of malaria vectors to insecticides used in vector control, complicates effects to reduce transmission rates.
- Economic factors lead to reliance on cheaper, ineffective treatments by poor individuals in malaria-endemic zones, promoting resistance.
- Weak public health infrastructure and intervention: Weak healthcare systems in endemic areas hinder effective surveillance, monitoring, and response to resistance patterns, making it difficult to implement appropriate treatment strategies.
- Global travel of individuals increases movement of people and goods can facilitate the spread of resistant strains across regions and countries, complicating control efforts.
- Environmental factors: changes in climate and land use can affect mosquito populations and malaria transmission dynamics, potentially influencing the spread of resistance.
Strategies To Minimise Antimalarial Drug Resistance
WHO strategy aims to minimize the threat and impact of antimalarial drug resistance in Africa through 4 pillars:
Strengthen surveillance of antimalarial drug efficacy and resistance
- Improve data dissemination systems to facilitate coordinated response to resistance data
- Enhance capacity to generate better quality and standardised data on antimalarial drug resistance and on parasite resistance.
Optimize and better regulate the use of diagnostics and therapeutics to limit drug pressure through pre-emptive measures
- Promote equitable access to quality drugs.
- Develop national treatment policies that promote the deliberate use of existing treatment to the emergence and spread of resistance.
React to resistance by limiting the spread of antimalarial drug-resistant parasites
- Limit the risk of increased transmission of resistant parasites.
- Ensure optimal vector intervention coverage in priority areas.
Stimulate research and innovation to better leverage existing tools and to develop new tools against antimalarial drug resistance
- Identify and develop innovative tools to limit malaria infection and transmission.
- Conduct modelling and research to better understand and track resistance. (6)
The challenge of antimalarial drug resistance is a pressing issue that requires immediate attention and action from all stakeholders involved in malaria control efforts. A multifaceted approach that includes developing new treatments, employing combination therapies, enhancing surveillance systems, and engaging communities will be crucial to overcoming these challenges hence, reducing the burden of malaria and improving public health outcomes globally.
Bibliographic References
- WHO. Malaria. 4 Dec. 2023, www.who.int/news-room/questions-and-answers/item/malaria.
- Sato, Shigeharu. (2021). Plasmodium—a brief introduction to the parasites causing human malaria and their basic biology. Journal of Physiological Anthropology. 40. 10.1186/s40101-020-00251-9. (PDF) Plasmodium—a brief introduction to the parasites causing human malaria and their basic biology (researchgate.net)
- WHO. 4 Dec. 2023, www.who.int/news-room/questions-and-answers/item/malaria.
- WHO. Malaria: Artemisinin Partial Resistance. 18 November 2022. Malaria: Artemisinin partial resistance (who.int)
- Sinha, Shweta & Medhi, Bikash & Sehgal, Rakesh. (2014). Challenges of drug-resistant malaria. Parasite (Paris, France). 21. 61. 10.1051/parasite/2014059.
- WHO.“Tackling emerging antimalarial drug resistance in Africa.” 18 Nov. 2022, www.who.int/news/item/18-11-2022-tackling-emerging-antimalarial-drug-resistance-in-africa.
- Menard D, Dondorp A. Antimalarial Drug Resistance: A Threat to Malaria Elimination. Cold Spring Harb Perspect Med. 2017 Jul 5;7(7):a025619. doi: 10.1101/cshperspect.a025619. PMID: 28289248; PMCID: PMC5495053.
- Oladipo HJ, Tajudeen YA, Oladunjoye IO, Yusuff SI, Yusuf RO, Oluwaseyi EM, AbdulBasit MO, Adebisi YA, El-Sherbini MS. Increasing challenges of malaria control in sub-Saharan Africa: Priorities for public health research and policymakers. Ann Med Surg (Lond). 2022 Aug 18;81:104366. doi: 10.1016/j.amsu.2022.104366. PMID: 36046715; PMCID: PMC9421173.
